Multimeric protein complexes are essential components of many cellular processes. These complexes consist of multiple proteins that work together to perform specific functions. To better understand these complexes, researchers use various techniques to identify and characterize the individual proteins that make up these complexes.
One powerful tool for studying protein complexes is tandem affinity purification-mass spectrometry (TAP-MS). This method involves the use of two affinity tags to isolate and identify protein complexes from cells.

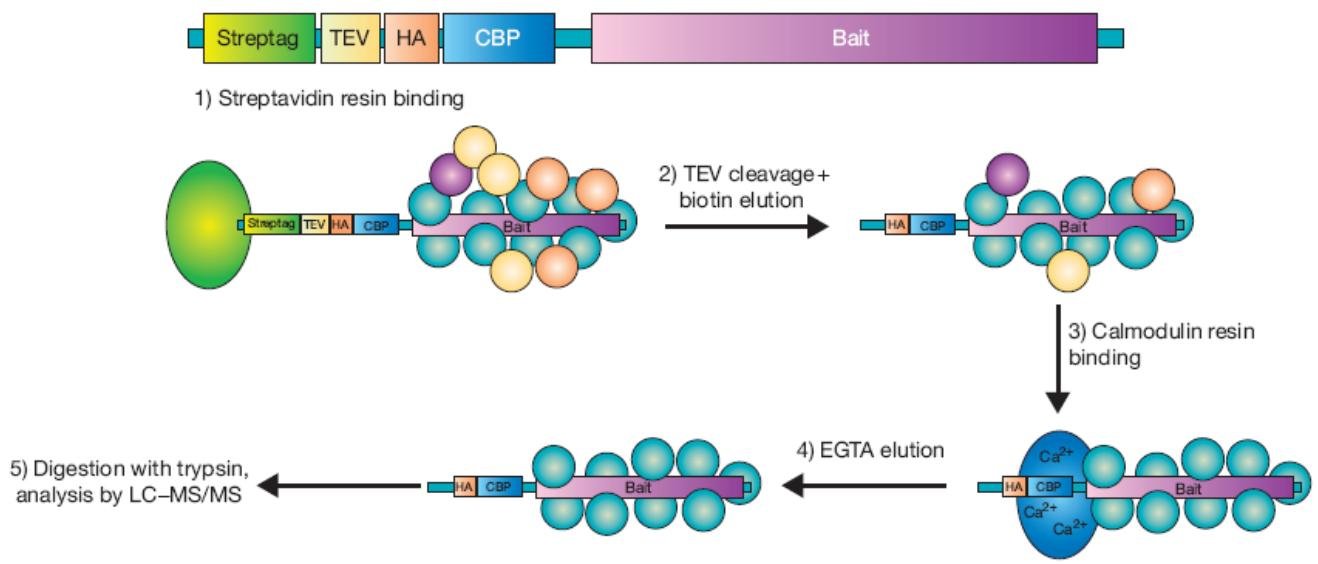
Schematic representation of the tandem-affinity purification strategy (Camp et al., 2012).
Tandem Affinity Purification (TAP)
The magic behind TAP-MS lies in the clever use of not one but two affinity tags, namely Protein A and calmodulin-binding peptide (CBP), that work synergistically to purify protein complexes. The first tag, Protein A, is a highly specific ligand that tightly binds to IgG beads, thus enabling the efficient capture of TAP-tagged protein and associated proteins from cell lysates. The TAP tag, a sophisticated genetic fusion protein containing a tobacco etch virus (TEV) protease cleavage site, permits the facile release of the protein complex from the IgG beads, thereby enabling downstream analysis. The second tag, CBP, is a versatile ligand that binds to calmodulin-coated beads in the presence of calcium ions, allowing for additional purification of the protein complex. By using calcium-chelating agents, the protein complex can be eluted from the CBP beads with exquisite precision, ensuring the identification of all components of the complex.
Mass Spectrometry
Upon successful TAP purification, the protein complex is now ready to undergo the cutting-edge technique of mass spectrometry (MS) analysis. MS, an avant-garde method, exploits the mass-to-charge ratio of ions created from molecules in a sample to yield invaluable insights into the nature of the protein complex.
In TAP-MS, the protein complex is first delicately separated by either gel electrophoresis or liquid chromatography, depending on the desired outcome. Then, the proteins within the complex are meticulously digested into peptides, a process that requires utmost precision and attention to detail. The resulting peptides are ionized, an electrical charge being bestowed upon them, and ushered into the MS instrument for analysis.
Inside the MS instrument, the peptides are separated based on their mass-to-charge ratio. The MS instrument generates a mass spectrum that visually represents the peptides in the sample, each peak in the spectrum corresponding to a peptide of specific mass-to-charge ratio. The resulting spectrum reflects the complexity of the protein complex.
The mass spectrum is then compared to vast protein sequence databases to identify the proteins in the complex. This step requires a keen eye for detail and a sharp mind, as the search can be highly intricate and convoluted. However, once successful, this final step of the TAP-MS analysis provides researchers with a treasure trove of information about the constituent proteins of the complex, unlocking the secrets of the underlying cellular processes that govern our lives.

Overview of the Basic Protocols (Adelmant et al., 2019).
Advantages of TAP-MS
TAP-MS is hailed by scientists as a revolutionary method for probing the intricacies of protein complexes. Compared to other techniques, TAP-MS offers a slew of distinct advantages.
- TAP-MS enables the purification of native protein complexes from cells, allowing for the preservation of their structural and functional integrity. This ensures that the purified complexes accurately reflect their in vivo behavior.
- TAP-MS is an extremely sensitive technique, capable of detecting low-abundance proteins within a complex. This attribute makes TAP-MS an invaluable tool for analyzing protein complexes that are difficult to isolate or purify by other means.
- TAP-MS can be applied across a range of organisms, from simple bacteria to complex multicellular organisms like mice. This versatility makes TAP-MS a valuable tool for comparative studies of protein complex composition and function across species.
Applications of TAP-MS
TAP-MS has been used to identify and characterize protein complexes from a range of organisms, including bacteria, yeast, and multicellular organisms such as Caenorhabditis elegans, Drosophila, and mice. The TAP-MS approach has also been used in a variety of biological research areas, including gene regulation, signal transduction, and disease pathways.
For example, the proteomic technique of tandem affinity purification-mass spectrometry (TAP-MS) has been deployed with remarkable efficacy in disentangling the intricacies of RNA-induced silencing complex (RISC) and spliceosome assemblies, both of which are involved in the gene silencing and RNA splicing mechanisms, respectively. Furthermore, this innovative methodology has been employed with utmost finesse to scrutinize the complex web of protein-protein interactions. By way of illustration, TAP-MS has been skillfully deployed to reveal the interactome of the E3 ubiquitin ligase parkin, which is a pivotal player in the pathogenesis of Parkinson’s disease. In addition, TAP-MS has also been utilized with exceptional acuity to decipher the interactome of the histone methyltransferase EZH2, which has been implicated in the oncogenic pathways of various cancers.
References
- Camp, Nathan Daniel. WTX is a novel regulator of ubiquitination in the Wnt/β-catenin and KEAP1/NRF2 pathways. University of Washington, 2012.
- Adelmant, Guillaume, et al. “Tandem affinity purification and mass spectrometry (TAP‐MS) for the analysis of protein complexes.” Current protocols in protein science 96.1 (2019): e84.
Read More: